Avoiding Tailing and/or Adsorption Phenomena on Jordi Gel Columns
Because of the large number of aromatic rings inherent in the packing’s structure Jordi Gel columns based on divinylbenzene will give unique responses to certain types of samples.
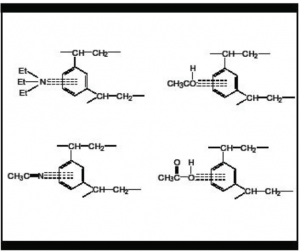
If your materials of interest contain aromatic rings or atoms such as O or N with unshared electron pairs, they have the potential to be strongly retained and/or tail on the Jordi Gel columns UNLESS there is a competing electron-rich solvent in the mobile phase.
Thus, to obtain sharper peaks with less tailing and good resolution, you can “adjust” the surface chemistry with a competing electron-rich solvent like acetonitrile or use a mobile phase additive such as triethylamine (TEA) or n-butylamine which can coordinate with the aromatic rings of the packing material creating a less electron-dense surface chemistry.
For certain separations it is also possible to use sodium acetate to modify peak shape and retention intensity. In like manner, using low percentages of glycerol, 2-propanol, or other similarly hydrophilic hydroxylated solvents reduces the net effective surface hydrophobicity.
The diagrams below indicate several possible electron interactions of mobile phase modi-fiers with the aromatic rings of the DVB gel to modify the surface hydrophobicity.
In our experience it is best to use quantities of 0.5-2.0% of TEA or ethylene glycol, or 0.01M Na Acetate, and anywhere from 2.0-100% of solvents such as CH<sub>3</sub>CN, CH<sub>3</sub>OH, or 2-propanol. We have also found that 50/50 V/V CH<sub>3</sub>CN/CH<sub>3</sub>OH mixture as strong solvent is better than either used alone.
For samples such as hindered amine light stabilizers containing the peperazine group, we have found that 98/2 V/V CHCl /TEA or 75/25 V/V THF/ MeOH with 0.01 M NaAc are excellent mobile phases and yield high quality GPC results on the Jordi Gel DVB base GPC columns.