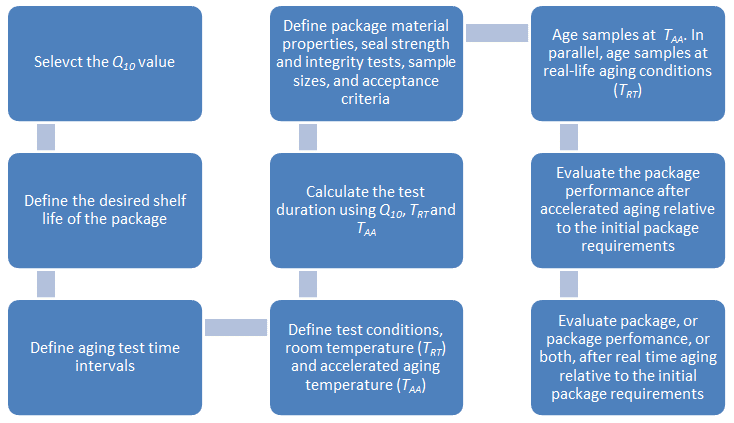
Accelerated aging is a process of holding packaged products at an elevated temperature to simulate real time aging in a reduced amount of time. It is utilized to aid in determining the specific expiration date for a medical device product or package, and it provides an alternative way to study new products compared to the real time aging, which requires longer periods of time which are often impractical. ASTM-F1980 is the widely accepted standard for accelerated aging testing. The guideline provides information for developing accelerated aging protocols to promptly determine the effects, due to the passage of time on the integrity of the sterile barrier system, and the physical properties of their component packaging materials. The figure below is an example of the results obtained for accelerated aging of polymers from the ASTM F1980 standard.
The ASTM F1980 procedure for accelerated aging is comprised of the following:
Reference:
http://file.yizimg.com/424653/2013120515134271.pdf




