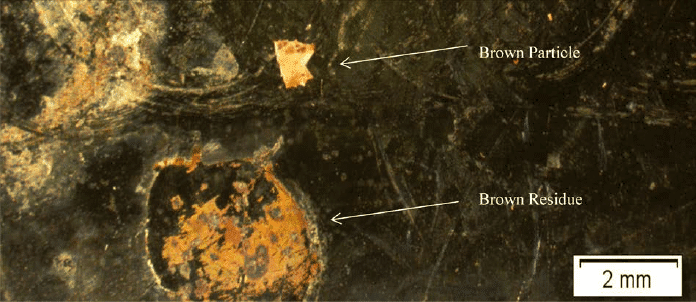
Quality assurance and control (QA/QC) are paramount in the pharmaceutical industry, where product contamination can have severe consequences to consumers of prescription drugs and patients in medical facilities. Among the most common and concerning contaminants in pharmaceuticals are particulates and residues.
Particulates and residues can be generated at virtually any stage of a product’s lifecycle, from initial raw material processing to storage of the drug/device. The underlying reason for this potential ubiquity is that particulates and residues can propagate from any number of sources: processing equipment, packaging, undissolved residuals, media solutions, virtually any system component, and much more. As a result, there is no such thing as a typical particulate or residue. They may be comprised of technically limitless materials, from glass to wood.
Identifying particulates and residues in pharmaceutical goods is essential for optimal QA/QC, to ensure patient health and unerring regulatory compliance. Once the source of contamination is detected, it can be eliminated from the manufacturing chain.
In this whitepaper, Jordi Labs explores the analysis of brown particulates and residues via Fourier transform infrared (FTIR) microscopy and scanning electron microscopy energy dispersive X-ray (SEM-EDX). Learn how these tools helped eliminate a source of rust form a pharmaceutical bioreactor, guaranteeing optimal processing conditions for future batch production.