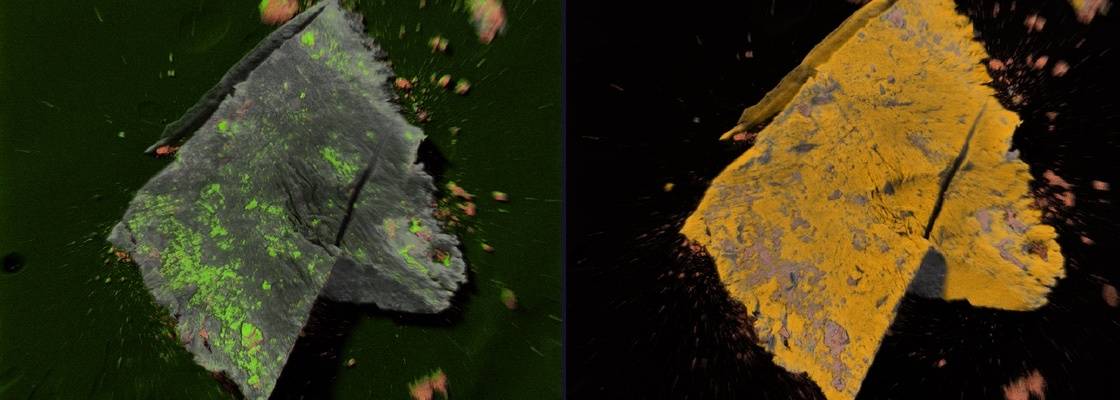
Particulates and residues are major types of contamination found in the pharmaceutical industry. These may be generated from a wide variety of sources including packaging, undissolved residuals in buffer and media solutions, and various system components such as gaskets and seals. Contamination can also result from side reactions related to the manufacture of the product, including charred products and detergent residues or from degradation or maintenance of the processing equipment such as metal corrosion, lubricant oils and scoring of Teflon gaskets. Particulates are found to be composed of a wide range of materials including glass, rubber, aluminum, plastics and wood-based products.
Particulates can spread to the surrounding air volume of a clean room through airborne contamination or by transport attached to people or containers. This can then result in contamination of both products and the manufacturing area. These particles, if carried over to the final drug product, can have unfavorable effects such as impairment, microcirculation, blockages of blood vessels and damage to various organs, phlebitis etc.
The FDA requires documentation and investigation of unexpected particles or adulterated drugs1, and has taken action against companies that fail to perform adequate investigations for violations involving particle contamination2. Methods for testing include:
- USP 787/788/789
- EN 45502
- ISO 14708
Identifying and understanding the source of particulates is critical to controlling their spread. Once the source is known, then elimination of the particulate contamination becomes substantially simpler.
Jordi Labs utilizes FTIR-microscopy, SEM-EDX, and other techniques to identify particles, as well as provide information about shape, size and surface topography.
1.FDA Regulation 21 CFR 211 Subpart E
2.FDA fines drug manufacturer $500 million for violations including insufficient investigation of rejected lots